|
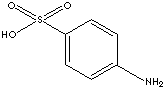
SULFANILIC ACID
| ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 121-57-3 |
|
| EINECS NO. | 204-482-5 | |
| FORMULA | (H2N)C6H4SO3H | |
| MOL WT. | 173.19 | |
| H.S. CODE | 2921.42.2200 | |
| TOXICITY | Oral rat LD50: 12,300 mg/kg | |
| SYNONYMS | 4-Aminobenzenesulfonic acid; p-Anilinesulfonic acid; Sulfanilic acid; | |
| Kyselina Sulfanilova (Czech); Sulfanilsaeure (German); Aniline-p-sulfonic acid; 4-Sulfanilic acid; Aniline-4-sulfonic acid; Other RN: 856062-06-1 | ||
| SMILES | S(c1ccc(N)cc1)(O)(=O)=O | |
|
CLASSIFICATION |
Benzenesulfonic acid, Aniline |
|
|
EXTRA NOTES |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | light grey powder | |
| MELTING POINT | 288 C (Decomposes) | |
| BOILING POINT | ||
| SPECIFIC GRAVITY | 1.485 | |
| SOLUBILITY IN WATER | 1 g/100 ml | |
| SOLVENT SOLUBILITY |
| |
| AUTOIGNITION | ||
| pKa | 3.34 | |
| VAPOR DENSITY | ||
| log P | -2.16E+00 (Octanol-water) | |
| VAPOR PRESSURE | 2.02E-07 (mmHg at 25 C) | |
| HENRY LAW CONSTANT | 8.89E-13 (atm-m3/mole at 25 C) | |
| OH RATE CONSTANT | 2.32E-11 (cm3/molecule-sec at 25 C Atmospheric ) | |
| NFPA RATINGS | Health: 1 Flammability: 1 Reactivity: 0 | |
|
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions | |
|
EXTERNAL LINKS & GENERAL DESCRIPTION |
||
|
Drug Information Portal (U.S. National Library of Medicine) - Sulfanilic acid PubChem Compound Summary - Sulfanilic acid KEGG (Kyoto Encyclopedia of Genes and Genomes) - Sulfanilic acid http://www.ebi.ac.uk/ - Sulfanilic acid http://www.ncbi.nlm.nih.gov/ - Sulfanilic acid http://infohost.nmt.edu/ http://www.akademiai.com/ Local: |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
light grey powder | |
| PURITY |
98.5% min | |
|
ANILINE |
0.3% max | |
|
INSOLUBLES |
0.1% max | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | ||
| UN NO. |
| |
| SAFETY INFORMATION | ||
|
HAZARD OVERVIEW |
May cause respiratory tract irritation. May cause allergic skin reaction. Causes eye and skin irritation. Target Organs: Skin |
|
|
GHS |
|
|
| SIGNAL WORD | Warning | |
|
PICTOGRAMS |
|
|
|
HAZARD STATEMENTS |
H319-H315-H317 |
|
|
P STATEMENTS |
P280-P302 +P352 |
|
| EC DIRECTIVES |
|
|
| HAZARD CODES |
|
|
|
RISK PHRASES |
36/38-43 |
|
|
SAFETY PHRASES |
24-37 |
|
|
|
||